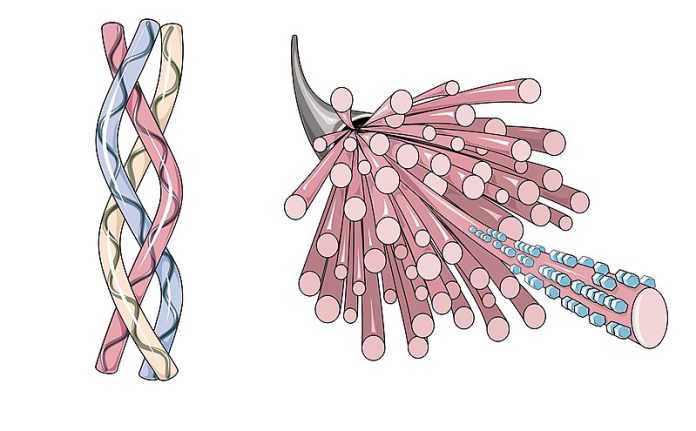
Healthremedy123.com – The structure of collagen is highly variable and can differ from protein to protein. For instance, collagen consists of two triple helical chains, one of which is the C-terminus. This is a characteristic of a triple helix. The sides of the helical chains are complementary in their hydrogen bonding, hydrophobicity, and opposite charges. In contrast, the stagger of the triple-helical chain is completely controlled by the NC2 domain. Consequently, the triple helical chain linked to the chain B is always in the leading position, while the one linked to the chain A is at the trailing position.
Repeating Sequence of Three Amino Acids
The triple helix in collagen is a repeating sequence of three amino acids, each with a different shape. The first three residues of this triple-helical sequence are glycine, which has a small side chain and forms an ideal elbow within the helix. The remaining two amino acids are proline or hydroxyproline, which is less common in collagen. The triple helix has a helix-like configuration because of the juxtaposition of the two amino acids.
One of the most interesting features of the NC2 domain of collagen is its high content of a helice in parallel organization. This property distinguishes this structure from a classical coiled coil. It also indicates a right-handed bundle of helices compared to the left-handed superhelix of classical coiled coils. However, the NC2 domain of collagen is more rigid than other types. Therefore, it is possible to study the structure of this type of collagen with high precision.
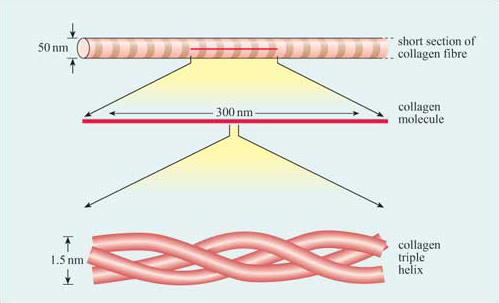
There are two ways in which collagen is synthesized: inside and outside of cells. The most common type of collagen is fibrillary, while meshwork collagen is used in filtration systems. Both collagen types are triple helices, with the difference lying in the structure of alpha peptides. This difference is important for the structure of the fibrils and its role in disease. So, when you want to understand the structure of collagen, start reading this article! You will be glad you did!
High Collagen Glycine Content
The high glycine content of collagen is important for its stabilization. It also enables the collagen fibers to be close together within the molecule. This helps facilitate hydrogen bonding and the formation of intermolecular cross-links. Only a few other proteins have this regular pattern of collagen fibers. Silk fibroin is another example of a high glycine content. This makes it easier to break down and digest in a cell.
The TC monomers of type I collagen are unstable at body temperatures. To stabilize the triple helices, collagen fibrillogenesis must be triggered. This process allows the formation of strong macromolecular structures that are able to withstand stresses. Kadler and coworkers concluded that collagen fibrillogenesis principles were established at least 500 Mya. And this means that they were formed long before our species evolved. And, as a result, we now know the exact shape of collagen fibrils.
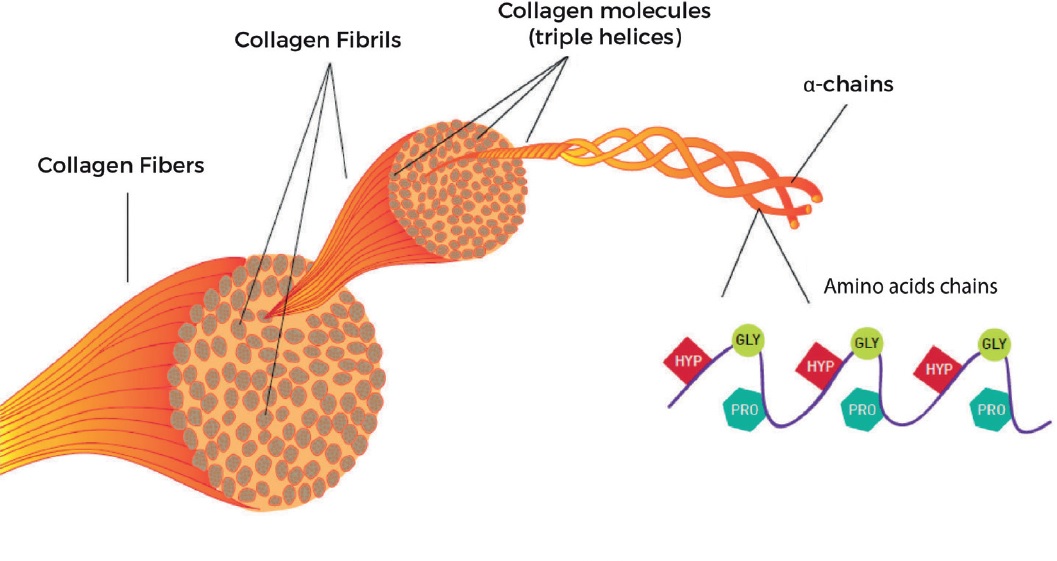
The structure of collagen is complex. It is made up of three polypeptide strands that are one hundred and thirty nanometers long. Each strand has a left-handed helix. However, do not confuse it with the right-handed alpha helix. The three left-handed helices then twist together to form the right-handed triple helix, a cooperative quaternary structure. This super-coil is also known as a collagen microfibril.
Collagen Chain Consists of Rare Derived Amino Acids
In collagen, the amino acids glycine are essential for the protein’s stability. In addition to these two amino acids, the collagen chains are composed of rare derivative amino acids that are essential for the formation of fibrils. The high Pro/Gly content of these structural proteins supports the hypothesis that they play important roles in the molecular evolution of the protein. Therefore, the presence of these two amino acids may be essential to the stability of fibril structure.
The pitch of collagen microfibrils is a key feature for the biological function of cells attached to fibrils. These cells bind to the extracellular matrix through transmembrane integrin protein complexes that have binding sites with cell cytoskeletons. The nanoscopic structure of collagen provides the cell with well-defined ligand binding sites. This nanoscopic structure can be altered by macroscopic stress on collagen and its extracellular matrix, causing a 10% strain on the triple helix of collagen. The resultant fibrils cannot be compared to those of natural collagen.
To quantify the density of collagen fibers, SHG is a laser-based quantitative imaging technique. The density of collagen fibers is a critical factor for the collagen packing structure. The higher the signal is, the more dense the fibers are packed. Therefore, the higher the density of collagen fibers, the higher the SHG intensity. If the SHG is high, the fibers are more dense, but not the same size. Hence, collagen density is the key to assessing the quality of human tissue.
Reference: